Some Of Eaukangen Kangen Water
Wiki Article
Eaukangen Kangen Water Things To Know Before You Get This
Table of ContentsThe smart Trick of Eaukangen Kangen Water That Nobody is DiscussingThe Facts About Eaukangen Kangen Water RevealedLittle Known Facts About Eaukangen Kangen Water.Some Of Eaukangen Kangen WaterFascination About Eaukangen Kangen Water
In other words, the chemistry of life is water chemistry. Water is a global, excellent solvent as a result of the significant polarity of the water particle and its tendency to form hydrogen bonds with other particles. One water particle, expressed with the chemical symbol water, consists of 2 hydrogen atoms and also 1 oxygen atom.
Oxygen, on the various other hand, contains 8 protons in its center with 8 electrons focusing on it. This is commonly received chemical symbols as the letter O surrounded by 8 dots representing 4 collections of paired electrons. The solitary hydrogen electron and also the 8 electrons of oxygen are the trick to the chemistry of life because this is where hydrogen and also oxygen atoms incorporate to form a water particle, or split to develop ions.
A hydrogen bond takes place when the electron of a single hydrogen atom is shown to one more electronegative atom such as oxygen that lacks an electron. In a water particle, 2 hydrogen atoms are covalently bound to the oxygen atom. Since the oxygen atom is bigger than the hydrogen's, its tourist attraction for the hydrogen's electrons is alike better so the electrons are drawn better right into the shell of the larger oxygen atom and also away from the hydrogen coverings.
The Basic Principles Of Eaukangen Kangen Water
Ionized water: Because the coverings of the hydrogen atoms are more detailed to the oxygen, their electrons take on a tiny electropositive charge. This indicates water molecules have a tendency to form weak bonds with water particles due to the fact that the oxygen end of the molecule is adverse and also the hydrogen ends declare - eaukangen kangen water.Similarly, the oxygen end of a particle can form a weak add-on with the hydrogen ends of various other molecules. Due to the fact that water molecules have this polarity, water is a continual chemical entity. These weak bonds play a vital duty in supporting the form of a lot of the big particles discovered in living issue.
In water the ionic bonds of the sodium chloride particle are damaged conveniently due to the fact that of the competitive activity of the countless water particles. As we can see from this basic example, also the delicate configuration of individual water particles enables them to break reasonably stronger bonds by converging on them.
The Of Eaukangen Kangen Water
It is an all-natural remedy that damages the bonds of bigger, extra complex molecules. This is the chemistry of life on earth, in water as well as on land. Essentially, decrease suggests the enhancement of an electron (e-), as well as its converse, oxidation suggests the elimination of an electron. The addition of an electron, reduction, shops energy in the decreased substance.
Whenever one compound is decreased, one more is oxidized. To clear up these terms, think about any type of two molecules, An and B. When molecules An and also B come right into get in touch with, right here is what occurs: B orders an electron from particle A. Molecule A has been oxidized due to the fact that it has actually lost an electron.
In organic systems, removal or addition of an electron makes up one of the most frequent system of oxidation-reduction reactions. These oxidation-reduction responses are frequently called redox responses. An acid is a compound that enhances the focus of hydrogen ions (H+) in water. A base is a compound that lowers the concentration of hydrogen ions, in other words, raising the concentration of hydroxide ions OH-.
The Only Guide to Eaukangen Kangen Water

At p, H 7, water contains equal focus of H+ and also OH- ions. Substances with a p, H much less than 7 are acidic due to the fact that they have a higher concentration of H+ ions. Compounds with a p, H more than 7 are alkaline because they consist of a higher concentration of OH- than H+. eaukangen kangen water.
Importance of harmonizing p, H Read More Here Living things are Extra resources very conscious p, H as well as feature best (with particular exemptions, such as particular parts of the digestion tract) when options are virtually neutral. A lot of interior living matter (excluding the cell core) has a p, H of concerning 6. 8. p, H in water: Blood plasma as well as other fluids that border the cells in the body have a p, H of 7.
3. Countless special mechanisms help in stabilising these liquids to ensure that cells will certainly not be subject to appreciable variations in p, H. Materials which work as systems to maintain p, H are called barriers. Barriers have the capacity to bond ions and also remove them from service whenever their focus begins to rise.
All about Eaukangen Kangen Water
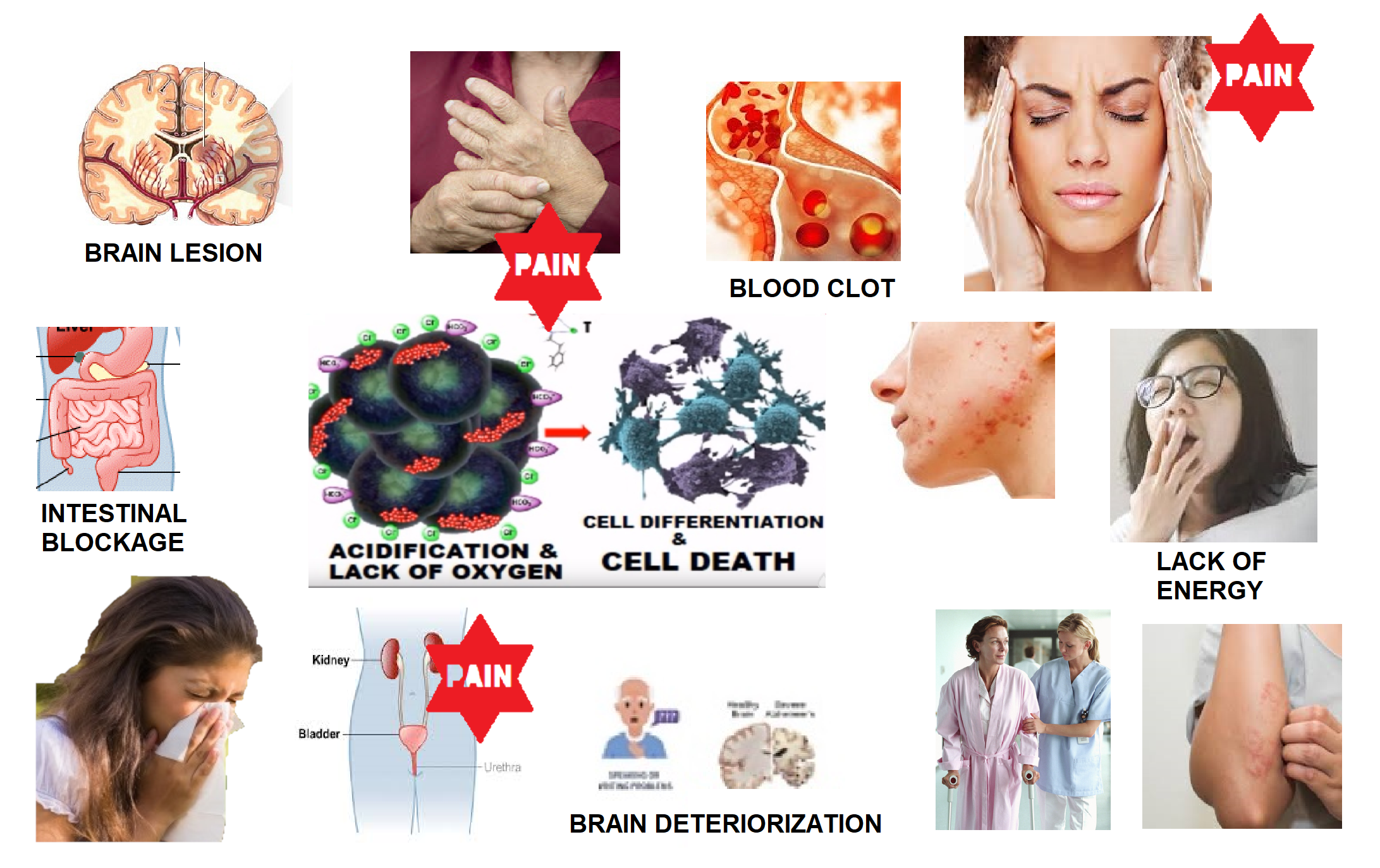
About 2% of the oxygen we usually breathe becomes active oxygen, as well as this amount increases to roughly 20% with cardiovascular workout Free radicals: Such free radicals with unpaired electrons are unpredictable and also have a high oxidation possibility, which implies they are capable of stealing electrons from various other cells. This chemical mechanism is very useful in disinfectants such as hydrogen peroxide and ozone which can More Bonuses be made use of to sterilize wounds or clinical instruments.If we can find an efficient approach to obstruct the oxidation of healthy and balanced cells by energetic oxygen, then we can try to avoid disease (eaukangen kangen water). One method to protect healthy and balanced cells from the ravages of oxidation triggered by energetic oxygen is to give totally free electrons to active oxygen radicals, thus counteracting their high oxidation capacity and also avoiding them from reacting with healthy cells.
Report this wiki page